Expanded Capabilities for Filtration Through new Filter Bag Assembly, Offering Comprehensive Upstream and Downstream Operational Solutions
After decades as a leading filtration supplier to the demanding semiconductor market, Entegris has applied our uncompromising commitment to quality and purity to helping customers in bioprocess manufacturing. The addition of a single-use, sterilizing grade, PES filter into our life sciences portfolio perfectly complements our existing single-use bioprocess assemblies.
With this portfolio expansion, we now leverage quick-turn design and custom assembly capabilities alongside filtration expertise to provide customers a comprehensive bioprocess solution for upstream and downstream operations.
Entegris offers filtration implementation and on-going support by helping select filters, troubleshoot, and/or scale bioprocessing steps. We are committed to providing continuous support for your single-use needs and integration into critical processing step.
Pharmsteri™ II PES 0.22 Buffer Capsule Filter
Our Pharmsteri II PES 0.22 buffer capsule filter is a PES 0.22 µm filter that offers customers superior flow performance. It maximizes throughput performance, enhances flow rates, provides adequate chemical compatibility, and is high quality for integration into single-use systems. These properties enable customers to decrease the frequency of filter changeouts, eliminate process interruptions, and improve filter lifespan. The filter is designed for the sterile filtration or bioburden reduction of process fluids in biopharma and pharma processes, including buffers, media, intermediates, and other non-fouling applications.
- The asymmetric PES membrane is sterilizing grade and meets bacterial retentiveness per ASTM F838-20, to ensure that microorganisms, such as bacteria, are removed from the fluid stream.
- Cutting-edge filter design has been optimized for pleat geometry and performance, thereby delivering superior flow rates in a broad range of chemical conditions.
- Gamma-stable materials of construction with 1.5” sanitary Tri-Clamp® connections allows easy integration into any customer-specific, single-use assembly.
- Comprehensive field and application support capabilities in Billerica, MA, Logan, UT, and Hangzhou, China.
- Autoclavable materials allow users to integrate filters in situ to meet their own specific templates and needs.
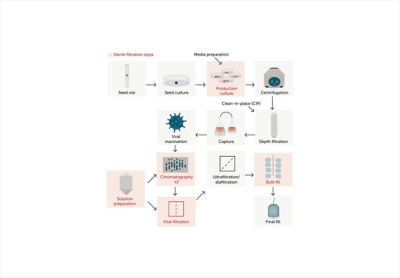
Manufacturing Process
Throughout the manufacturing process, Entegris has applied a high level of quality controls to ensure the production of each device with top-tier quality.
Pharmsteri™ II PES 0.22 buffer capsule filters provide high throughput performance with excellent biocompatibility during upstream or downstream bioprocesses wherever sterile filtration is needed.
We offer best-in-class lead times, meaning we can have a filter at your door in a matter of days or weeks.
Technical Paper
Vendor Partnering in a Bioprocess Manufacturing and Supply Chain Ecosystem
This technical paper explains how partnering with a vendor experienced in navigating regulations and validating new materials can help safeguard your treatments in a bioprocess manufacturing and supply chain ecosystem.
Featured Report
From concept to market: How to improve manufacturing process efficiency to increase speed-to-market
This Pharma IQ report looks at the ways pharmaceutical companies can improve their manufacturing processes and make efficiencies, from the concept stage through to market.

Featured Webinar
BioProcess Online | Sterile Filtration and Optimization for Single-Use Integration in Life Sciences
Chris discusses the value of optimizing sterile filtration by design and how it integrates into single-use systems and maximizes performance.
Key learning objectives:
- Better understanding of manufacturing material optimization
- Understand how membrane and capsule configuration designs maximize performance
- Learn how filter designs can be integrated seamlessly into single-use systems
Featured Product
PES 0.22 µm capsule filter that delivers superior flow rates in a broad range of chemical conditions to deliver significant economic advantages.
- Gamma stable and autoclavable materials of construction enable sterilization using caustics or autoclaving, or for optimal bioprocessing, integrate into single-use assemblies for gamma irradiation
- Best-in-class flow rates meet or exceed other commercially available filters allowing increased volumes to be processed in a limited time
Quick-turn custom manufactured tubes and manifolds for single-use, bioprocessing systems.
- Personalized, timely, and useful attention ensure the solution meets your exact process needs, fast.
- Consistently fast development and delivery minimize the risk of production delays
- Nominal bag volumes, number and locations of bag ports, diptubes, hanging rods, filter meshes, any supplier's component, even customer-supplied components are all options.