Introducing our Sterile Filtration Solutions Portfolio
Entegris has been a leader in materials sciences and challenging filtration applications for over 30 years. Our teams possess unparalleled research and metrology capabilities to serve the world’s most demanding industries. Entegris manufactures filtration products capable of removing contaminants as small as three nanometers, along with exacting controls on metrology and purity. This materials science expertise has been applied with the launch of our new Life Science Filtration portfolio, which includes the single-use Pharmsteri™ capsule product line that has been optimized for biopharmaceutical manufacturing.
Learn how to select sterile filtration solutions for single or multi-use life science applications with Cris Rios, Senior Field Applications Engineer.
Webinar
Best Practices for PUPSIT Assembly Design and Operation
Sterile filtration is a critical final step in drug manufacturing, ensuring that liquid-based drug substances are free from microbial contaminants. In this webinar, discover how to design and implement Pre Use Post Sterilization Integrity Test (PUPSIT) assemblies to meet regulatory expectations and reduce operational risks.
eBook
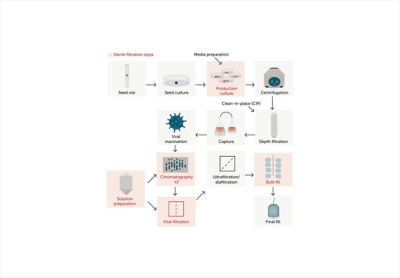
A Practical Guide to Single-Use Filtration in Biopharma
Materials purity is a key driver behind the revolution in single-use assemblies for biopharmaceutical applications. How can you select the right filter to remove microorganisms and larger contaminants in your application? The experts at Entegris bring you a complete guide to filtration for life sciences applications, answering questions such as how to optimize flow rates, minimize downtime, and ensure regulatory compliance.
Filtration Portfolio for Life Sciences
Our solutions address prefiltration and sterile filtration needs across diversified applications:
Pharmaceutical
- Monoclonal antibodies (mAb)
- Vaccines
- Plasma
- Active pharmaceutical ingredients (APIs)
- Large volume parenteral/Small volume parenteral (LVP/SVP)
Laboratory
- Lab analysis
- Environmental monitoring
Newest products with global regulatory compliance, delivered within six weeks
Delivers superior airflow at low pressure drop with lower blockage risk in moist air sterile filtration
Delivers superior throughput for broad challenging feed streams while minimize product loss in sterile filtration
PES 0.22 µm capsule filter that delivers superior flow rates in a broad range of chemical conditions to deliver significant economic advantages
Provide safe, sterile filtration
- Allows high flow and low pressure drop
- All components meet USP <88> Class VI Plastics tests
- Comprehensive validation package available as fast as eight weeks
- 100% integrity tested during manufacturing
- Compatible with most stainless-steel housings
Applications
- Pure water/water for injection
- Large volume parenteral/Small volume parenteral (LVP/SVP)
- Ophthalmic solutions
Efficiently remove particulate and colloidal contaminants
- All components meet USP <88> Class VI Plastics tests
- Lowers cost of ownership
- Allows high flow and low pressure drop
- Excellent chemical compatibility, allowing use in a broad range of applicationsApplicationsLarge volume parenteral/Small volume parenteral (LVP/SVP)
Applications
- Large volume parenteral/Small volume parenteral (LVP/SVP)
Global R&D Presence
i2M Center for Advanced Materials Science
Staffed with membrane and purification specialists with a wide spectrum of expertise in membranes, monoclonal purification, and surface modification. The i2M Center is the home to state-of-the-art research and development from lab to pilot scale, and prepares for membrane and purification production at our facilities in the United States and China.
Life Sciences Technology Center
Provides application testing and process development for bioprocess technologies including freeze/thaw and filtration applications to solve complex process problems before they occur.
Hangzhou Facility
Opened in 2020, this 33,000 m2 new facility provides filtration manufacturing and support services for life sciences.
Membrane R&D
New filtration membrane technologies are investigated and developed locally for life sciences filtration needs.
Product R&D
Local testing, analysis, and pilot capabilities allow rapid prototype development to improve total cost of ownership and reduce time to market.
Validation Services
Comprehensive validation services ensure filter specifications and performance are within international regulatory requirements.